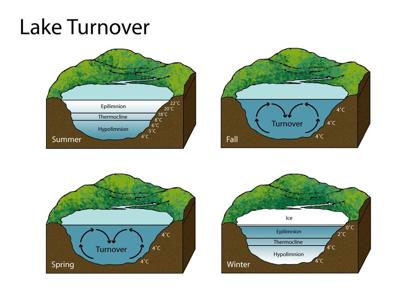
Have you ever noticed some lakes freeze while others don't? Think about the pond in your subdivision compared to the Great Lakes in the middle of winter.
When a body of water freezes it's not necessarily about the depth of the lake but about how much water the lake is holding. It all comes down to density. When everything is liquid water (during the warmer months), warmer water is less dense than colder water so the warmer water is at the top. When there's ice involved, though, the frozen state of water is actually less dense than the liquid water. So the ice rises to the top and the warmer water flips to the bottom. While really shallow bodies of water can freeze all the way through, most only freeze at the top.

Illustration by Tim Gunther via National Geographic
Since there is more at play here than just dropping below 32 degrees, that is not the magic number to freeze your lake. As the air temperature drops, the water temperature drops more slowly. As the water temperature drops through the 30s (Fahrenheit), the process described above starts to take place. Your lake or pond won't fully freeze when the water temperature drops below 32 degrees. You should also consider what outside factors may be affecting your body of water. How much sunshine does the area get? Is there new water flowing in or out or is this a stagnant body of water? So the question "why do some lakes freeze and others don't" is more complicated to answer than just pointing to one factor.
The Great Lakes are so large, do they freeze? Yes, even with so much water, ice does build up on each of the Great Lakes during different parts of the winter! In fact there's a whole website about the ice coverage of the lakes as it changes during the season.