LOUISVILLE, Ky. (WDRB) -- For some, the weight loss drug Ozempic is life-changing.
The popular weight-loss drugs known as GLP-1 receptor agonists, which were originally meant to treat diabetes, surged in popularity after the U.S. Food and Drug Administration approved them for weight loss in 2021. Now, they're all over the place — celebrities, TV advertisements, social media, news media, your neighbor.
It seems great. But for other users, it's a nightmare.
Lucinda Mason, a Mt. Washington resident, said her experience with the drug has been overwhelmingly positive.
"People even treat me differently now that I've lost weight," Mason said. "People tend to be friendlier and they tend to more accommodating. There's that perception that overweight people are lazy, that whole stereotype. They're not. I've got a different job (and) I make more money — things like that — since losing the weight."
But for Jacqueline Barber, a Louisville resident, Ozempic has led to severe health complications.
"I lost about 17 pounds from being sick and not having any nutrition," Barber said.
A Growing Investigation
WDRB began investigating Ozempic and other diabetes and weight loss drugs after learning of nationwide lawsuits, some of which include patients in Kentucky.
Barber, who took Ozempic starting in 2021 to manage steroid-induced diabetes, has been in and out of the hospital since then. She was hospitalized recently with severe blood clots and was diagnosed with gastroparesis, a condition that prevents the stomach from emptying properly.
"I want everybody to know what it did to me, what it could do to you," Barber said.
Since WDRB first reported her story last June, people from around the world have reached out to Barber to share similar experiences.
"My sister was recently diagnosed with gastroparesis," one message to Barber read. "She has lost over 100 pounds and is in the emergency room at least once every two weeks."
Barber's weight plummeted to 87 pounds before doctors took her off Ozempic, believing it was the cause of her condition, two years after she starting taking it.
Her home is now filled with medical supplies and prescriptions.
Mason, however, said Ozempic has dramatically improved her health.
"I went to the doctor for diabetes management in July 2022 because I was feeling pretty bad," Mason said. "I weighed about 260 pounds."
Since starting Ozempic, Mason's weight, cholesterol and A1C levels have all improved.
But the lawsuits against the manufacturers of Ozempic, Wegovy, Mounjaro and similar medications claim there are inadequate warnings about potential side effects, including gastroparesis. Barber's attorney, Andrew Vans Arsdale with the AVA Law Group, said the agency represents 5,000 clients nationwide dealing with the illnesses from those drugs. Of those, 135 cases are in Louisville, and another 270 cases are in Kentucky and Indiana statewide.
"It's a very slow process," Barber said. "There are so many people involved now, and it's multi-district litigation in Pennsylvania."
Novo Nordisk, the maker of Ozempic and Wegovy, defended the safety of its products in a statement to WDRB Investigates.
"Novo Nordisk believes that the allegations in these lawsuits are without merit, and we intend to vigorously defend against these claims. Patient safety is our top priority at Novo Nordisk, and we work closely with the US Food and Drug Administration to continuously monitor the safety profile of our medicines. GLP-1 medicines have been used to treat type 2 diabetes (T2D) for more than 19 years, and for the treatment of obesity for almost 10 years. This includes Novo Nordisk GLP-1 products such as semaglutide and liraglutide that have been on the market for more than 13 years. Semaglutide has been extensively examined in robust clinical development programs, large real world evidence studies and has cumulatively over 9.5 million patient years of clinical experience."
Eli Lilly, which manufactures Mounjaro released this statement, "Patient safety is Lilly's top priority, and we actively engage in monitoring, evaluating, and reporting safety information for all our medicines. Our FDA-approved label clearly warns that Mounjaro may be associated with gastrointestinal adverse reactions, sometimes severe. The label further states that Mounjaro has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis, and is therefore not recommended in these patients. These risks were communicated to and widely known by healthcare providers. We are vigorously defending against these claims."

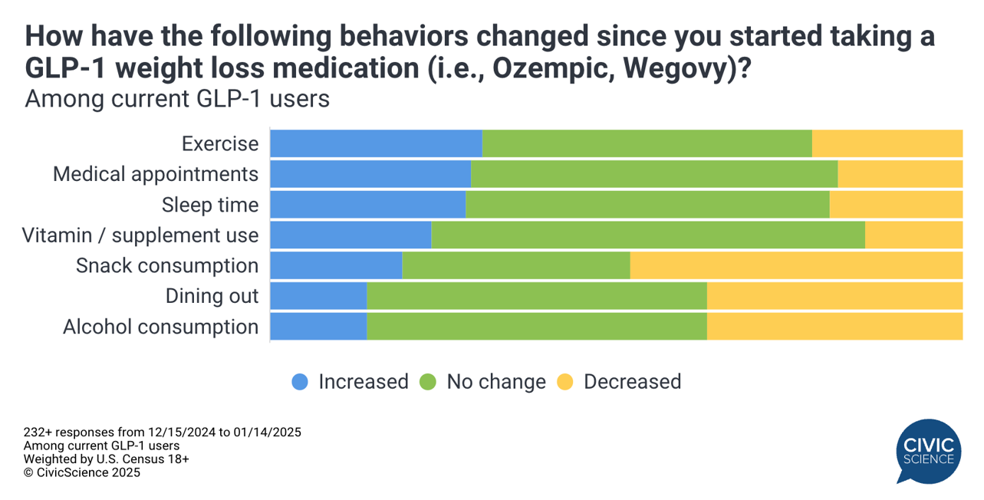
(Data courtesy of Civic Science)
A Divisive Drug
Dr. John Oldham, a bariatric surgeon with Baptist Health, said for 20% of patients taking Ozempic and similar prescriptions, the side effects are severe enough that they stop taking the drug altogether.
"The medications work by slowing stomach emptying, which is why some patients experience nausea, heartburn and vomiting," Oldham said. "But extreme cases like this are very rare."
Despite the lawsuits and health concerns, many people continue to swear by Ozempic. On WDRB's Facebook page, more than 250 people shared their experiences.
"I took semaglutide (Ozempic) short-term to kickstart weight loss," one commenter wrote. "I lost 80 pounds and have been off it for a year with no weight gain and no major side effects."
Others had more troubling experiences.
"I couldn't tolerate it," one person wrote. "To this day, I still have stomach problems from taking it."
Barber has very few things she can even eat when she's not using her IV, like crackers and granola bars. She continues to struggle with health issues, including ongoing blood clots that require extended use of blood thinners.
"If you are taking it — or new to taking it — and you get sick, please talk to your doctor," Barber said. "I really hate to see anyone end up where I'm at. It's so depressing. It really is."
Mason, who has lost 100 pounds and kept it off, stands by her decision to use the drug.
"It works," she said. "Occasionally, I have stomach issues. But, for me, getting my A1C and cholesterol down outweighed the risks."

The popular weight-loss drugs known as GLP-1 receptor agonists, which were originally meant to treat diabetes, surged in popularity after the U.S. Food and Drug Administration approved them for weight loss in 2021.
Copyright 2025 WDRB Media. The Associated Press contributed to this report. All Rights Reserved.