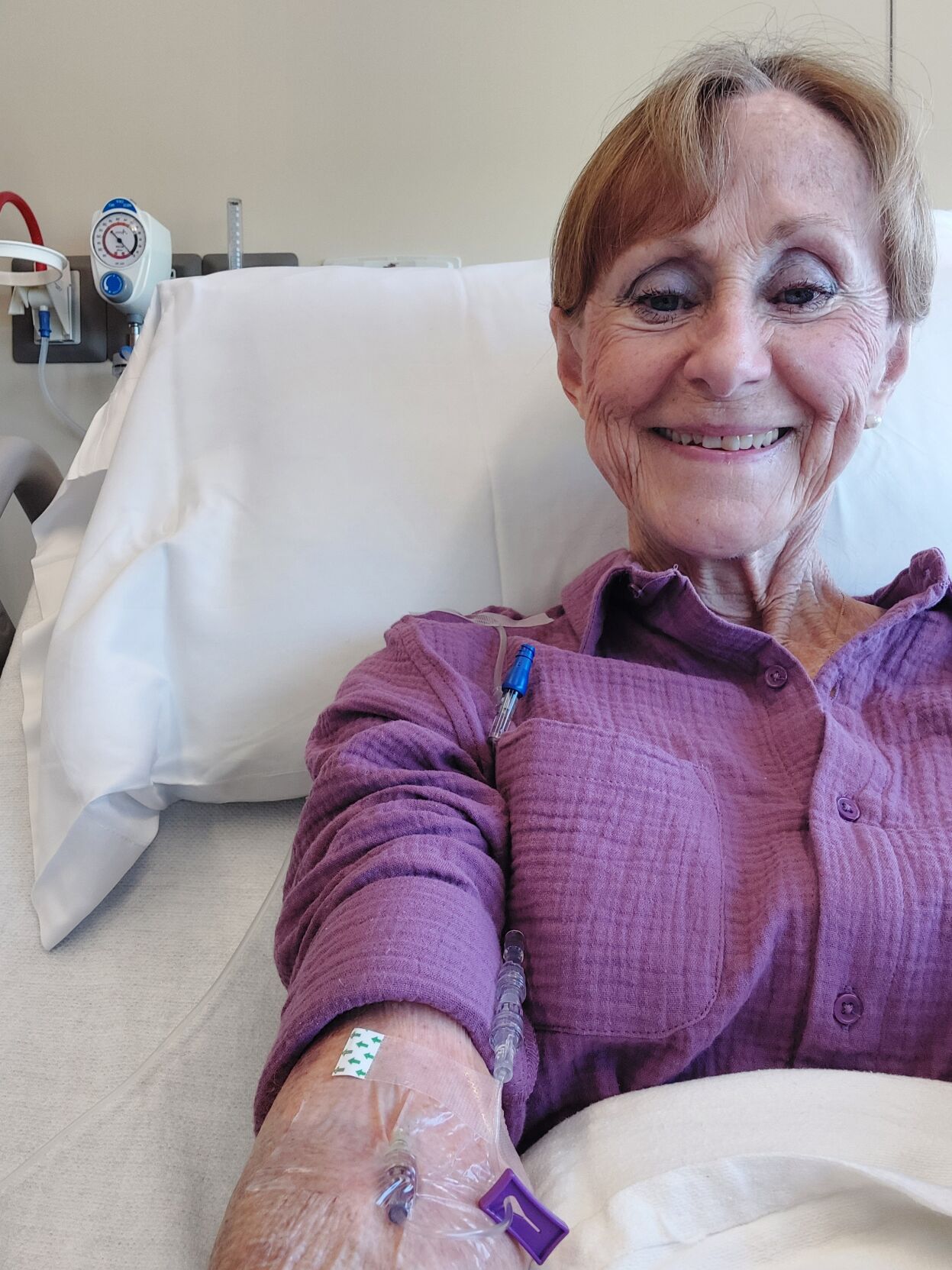
LOUISVILLE, Ky. (WDRB) -- Judy Miller has always had a sunny disposition. The 75-year-old Louisville woman who says she doesn't have a bad day put her life on hold when she was diagnosed with early on-set Alzheimer's in October 2022.
"Why me?" she said Thursday, remembering the aftermath of the diagnosis. "All the things I was going to do and had plans for were going to fall to the wayside."
But when she thought all hope was lost, things changed.
The U.S. Food and Drug Administration granted full approval in January 2023 of the IV drug Leqembi for patients with mild dementia and other symptoms caused by early Alzheimer's disease. It's the first medicine that's been convincingly shown to modestly slow the cognitive decline caused by Alzheimer's.

FILE - This Dec. 21, 2022, image provided by Eisai in January 2023 shows vials and packaging for their medication Leqembi. Leqembi, the first drug to show that it slows Alzheimer’s, was approved by the U.S. Food and Drug Administration in early January 2023. (Eisai via AP, File)
Instead of taking a pill like all previous treatment options, Leqembi requires Miller to simply go to the hospital for a transfusion every other Wednesday.
"Honestly, I got to tell you, I'm looking forward to it," she said. "I might wear my PJs next time."
Data from the Alzheimer's Association shows 75,000 people 65 and older live in Alzheimer's in Kentucky. In Indiana, 110,000 people 65 and older live with the disease. In both states, more than 11% of all residents aged 45 and older have some form of subjective cognitive decline.
Without insurance, Leqembi cost more than $26,500 for a year's supply of IVs every two weeks. But with Medicaid and Medicare on board to cover the treatment, private insurance companies could soon follow. That would expand access that's greatly needed, according to Shannon White with the Alzheimer's Association Greater Kentucky and southern Indiana Chapter.
The organization said more than 100,000 people in Kentucky and southern Indiana are currently living with Alzheimer's.
Japanese drugmaker Eisai received conditional approval from the FDA in January based on early results suggesting Leqembi worked by targeting the amyloid or "sticky" brain plaque linked to the disease. The FDA confirmed those results by reviewing data from a larger, 1,800-patient study in which the drug slowed memory and thinking decline by about 5.3 months in those who got the treatment for 18 months, compared to those who got a placebo.
The treatment isn't a cure, but it could buy people like Miller more time.
"I am so grateful for life and having more to come," she said Thursday. "... I do have a life sitting in front of me now."
Related Stories:
Copyright 2023 WDRB Media. The Associated Press contributed to this report. All Rights Reserved.